Abstract
Introduction: The association of malignancy, largely solid tumors, with venous thromboembolism (VTE) is well-known and a widely validated score, Khorana score, guides prophylactic anticoagulation in high-risk patients. However, increasing albeit scarce evidence suggests a high incidence of VTE in patients with hematologic malignancies, which are comparable to high-risk solid tumors. In this study, we sought to explore the incidence and predictors of VTE (including the variables in the Khorana score) in patients with acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) from a large database.
Methods: We performed a retrospective cohort study of patients diagnosed with AML or ALL using an aggregated de-identified data from electronic medical record of >300 major hospitals in US (IBM Watson Explorys). Patients aged 20 to 79 years, diagnosed with AML or ALL within the past 5 years were included. The primary endpoint was the incidence of cancer associated VTE defined as deep vein thrombosis, pulmonary embolism or superficial vein thrombosis within 1 year of AML/ ALL diagnosis. Baseline characteristics including age, sex, race, body mass index (BMI), prothrombotic mutations (Factor V Leiden, prothrombin gene 20210A mutation), smoking status, prior history of VTE, presence of central venous catheter (CVC), antineoplastic agent use, erythropoietin (EPO) and pegylated asparaginase (PegA) use as well as baseline laboratory values (as included in Khorana score) were compared between acute leukemia patients with cancer associated VTE within 1 year to those without. Fibrinogen and D-dimer were not available to be explored. A univariate analysis was performed using two-sided chi-square test to study these baseline variables. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA).
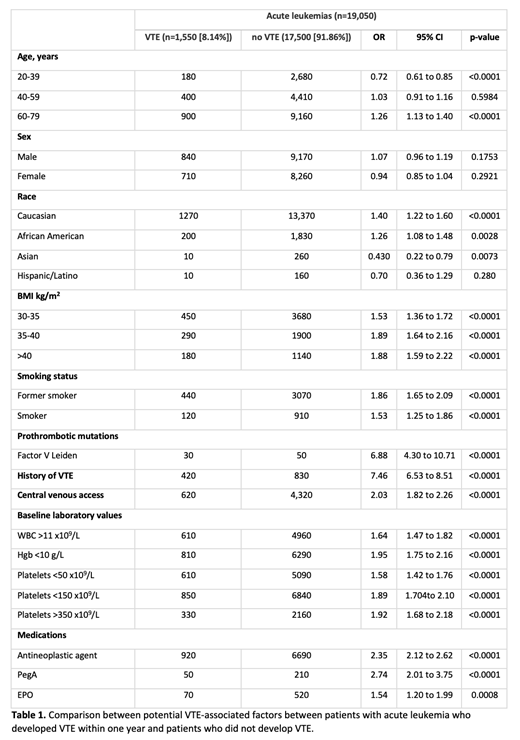
Results: A total of 19,050 adult patients with a diagnosis of AML or ALL in the last 5 years were included. Of these, 1,550 patients had VTE within 1 year of acute leukemia diagnosis, with an overall incidence of 8.14%. Baseline characteristics of patients with acute leukemia with and without cancer associated VTE within 1 year were compared (Table 1). On univariate analysis, age>60, Caucasian or African American race, obesity, remote or current smoking status, Factor V Leiden, prior history of VTE, presence of CVC, anemia, leukocytosis, antineoplastic agent/EPO or PegA use were all associated with an increased risk of cancer associated VTE. Interestingly, both low platelet counts (<50 x 10 9/L) and high platelet counts (>350 x 10 9/L) were associated with increased cancer associated VTE. Among these, prior history of VTE had the strongest association (OR 7.46). Younger age (20-39) and Asian race were in fact inversely associated with VTE. A multivariable analysis is currently underway to assess the significance of these risk factors independently towards cancer associated VTE in this AML/ALL population.
Conclusions: Our study highlights and reiterates a high risk of VTE within one year of diagnosis of AML/ALL in what we believe is the largest cohort studied to date. Older age, Caucasian or African American race, smoking, prior VTE, presence of CVC, Factor V Leiden, antineoplastic agent or PegA use were associated with increased risk. Among the Khorana score variables, obesity, anemia, EPO use and leukocytosis stood out as potential risk factors for VTE in this population. Risk of VTE was however increased regardless of platelet counts, which emphasizes the need to consider surveillance or thromboprophylaxis strategies in AML/ALL patients with thrombocytopenia with presumed high bleeding risk. Lastly, young age and Asian ethnicity seem to confer some protection against thrombosis in this population. Our ongoing efforts include a multivariable analysis and examining the effect of the Khorana score risk stratification in our large cohort of AML/ALL patients. Finally, further studies will be fundamental to meet the unmet need for a specific risk stratification system(s) for patients with AML/ALL.
Nayak: BioChip Labs: Current Employment.